
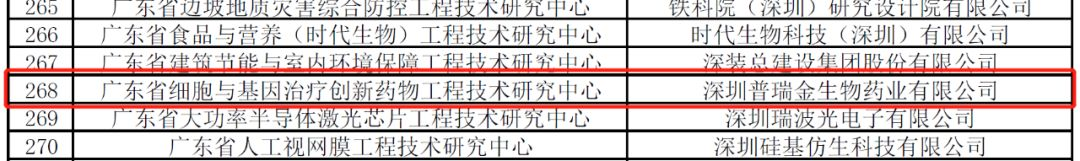
On March 5, 2020, the Department of Science and Technology of Guangdong Province issued the Notice of the Department of Science and Technology of Guangdong Province on Identifying the Engineering Technology Research Center of Guangdong Province in 2019, which was established by Pregene and jointly built by Shenzhen Children 's Hospital of Guangdong Lewwin.

Shenzhen PREGENE, which has been ploughing into the cell and gene therapy industry for years, has been successively recognized as a national high-tech enterprise and Zhongguancun high-tech enterprise, and its main research topic in this industry is CAR-T cell drugs (chimeric antigen receptor T cell drugs) and TCR-T cell drugs (T cell receptor T cell drugs) for the treatment of tumors. At present, two cell and gene therapy drugs for the treatment of cancer have submitted and accepted the application for registered clinical trial (IND) to China Food and Drug Administration according to national Class I new drugs. BCMA CART cell drugs for the treatment of multiple myeloma have shown the efficacy in the carried out non-registered clinical trials to reach the international leading level.
Joint applicant Guangdong Lewwin, a leading enterprise in the field of non-clinical evaluation and research of drugs in South China, GLP leading institution of non-clinical evaluation and research of drugs in South China, is the only institution in South China that has passed the NMPA (former CFDA) GLP certification at one time (9 items), and the first drug non-clinical evaluation and research institution in South China that has passed the international AAALAC full certification of a variety of experimental animals (mice, rats, guinea pigs, rabbits, dogs, etc.) at the same time in the field of pharmacology, toxicology, pharmacokinetics, new drug screening study and cell molecular biology research business.
Joint applicant Shenzhen Children 's Hospital - a modern comprehensive children' s hospital and pediatric emergency center integrating medical treatment, health care, scientific research and teaching, provides health care services for children in Shenzhen and more than a dozen surrounding areas. In 2017, it was rated as a Grade III Grade A pediatric general specialized hospital. The hospital has a total construction area of nearly 170,000 square meters and 1,300 open beds. Hospitals have more than 540 million advanced diagnostic and treatment equipment. Project carriers such as "Public Service Platform of Drug Clinical Trial Base", "Public Service Platform of Molecular Medicine for Pediatric Hematology and Oncology in Shenzhen" and "Key Laboratory for the Diagnosis of Severe Diseases in Children in Shenzhen" have been approved successively.
This center is built to further strengthen the innovative R&D of common key technologies industrialized in the field of cell and gene therapy drugs and the transformation of scientific and technological achievements, and build a whole-process platform for cell and gene therapy drugs integrating drug discovery, process development, toxicological safety evaluation, IND application, clinical trials and commercial production, which will effectively promote the development of cell and gene therapy drug industry in Dawan District, Guangdong, Hong Kong and Macao.
