Introduction to CAR-T Cell Agents
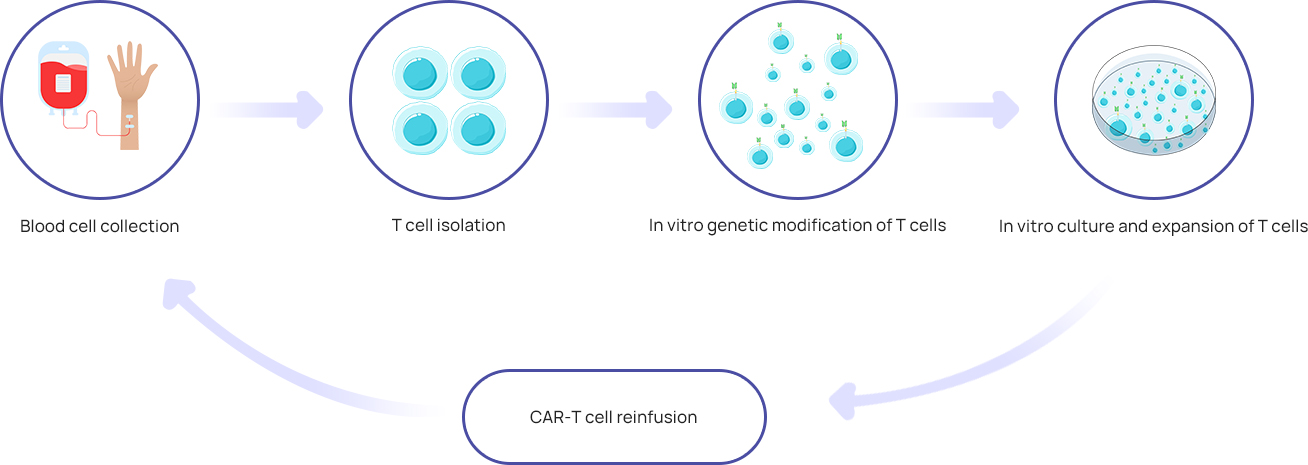
CAR-T cells, i.e., chimeric antigen receptor T cells, are based on the principle that patients' own T lymphocytes are re-engineered in the laboratory, loaded with receptors and costimulatory molecules that can recognize tumor antigens, expanded in vitro, and then reinfused into patient’s body, so as to recognize and attack the patient’s own tumor cells.
The extracellular antigen-binding region of CAR-T is derived from monoclonal antibodies and nanobodies.
In 2017, two CAR-T cell agents were approved by the US FDA for marketing, Kymriah developed by Novartis and Yescarta developed by Gilead/Kite, which have since opened a new era of immune cell therapy for tumor cells.